About Us
Company Profile
Established in 2017, Jiangsu Embrace Science &Technology Development Co., Ltd., strived to be at the forefront of the medical device industry by providing innovative world class medical disposable devices, and nursing equipment.
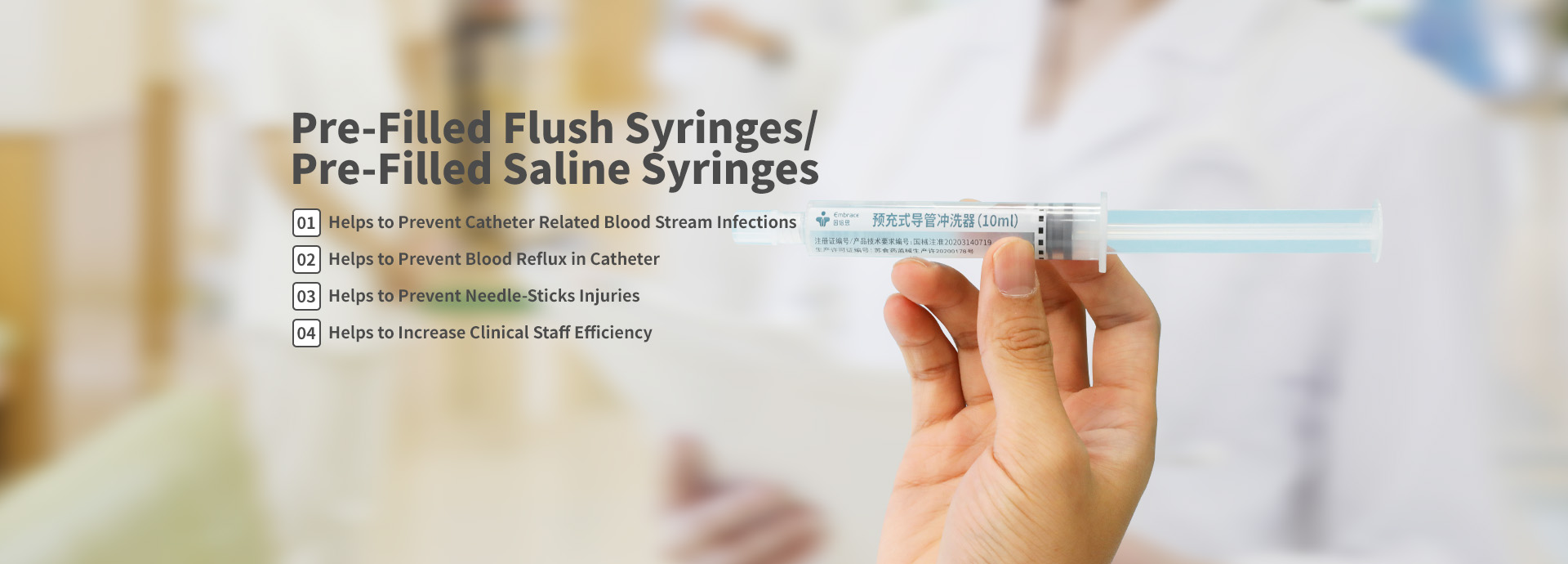
At present, our main manufacture products are pre-filled normal saline syringes, ranges covering 3mL, 5mL, 10mL.
The applications of our Embrace pre-filled normal saline includes flushing and locking of all kinds of Vascular Access Devices and Catheters, e.g. Peripheral Intravenous Catheters PIVC, Central Venous Catheters CVCs, Peripherally Inserted Central Venous Catheters PICCs, Implanted Infusion Ports such as Port-a-Cath, Haemodialysis Catheters, Umbilical Catheters, Arterial Catheters etc.
The company is located in the Huai'an Economic Development Zone, with convenient transportation. We have a technical team of experienced staff dedicated to strict quality control and attentive customer service. The company's production scale has been continuously expanded, and a series of advanced equipment such as hot runner injection moulding machines and automatic assembly machines have been introduced.
Embrace pre-filled manufactured in sites where the Quality Systems meet the strict requirements of the EU authorities (ISO 13485) with high sterility assurance of SAL10‾6. All sites are frequently audited by the regulatory agencies and their authorised agents. The manufacturing and testing environments are regularly monitored to demonstrate compliance to specifications and current GMP (Good Manufacturing Practice).
Because of the strict implementation of pharmaceutical GMP, medical device GMP and ISO 13485 management regulations, the company has established a sound organization. The product has passed CE certification. The QC department has complete testing equipment and complete testing centre, equipped with sample management room, chemical analysis room, physical and chemical room, standard liquid calibration room, balance room, common instrument room, gas chamber, infrared chamber, liquid chromatography room, and particles. The endotoxin testing room, high greenhouse, microbial limit testing room, sterilizing test room, positive control room, preparation room, stability test room, sample observation room, etc., and have laid a solid foundation for ensuring product quality.